
Mechanical Fragility as a Potential Time-Independent Measure of Membrane Integrity among Stored RBC Units Tarasev M1, Alfano K1, Chakraborty S1, Bertholf M2 and Zubair A3*
1Blaze Medical Devices, Ann Arbor, MI, USA 2The Blood Alliance, Inc., Jacksonville, FL, USA 3Mayo Clinic, Jacksonville, FL, USA
Abstract
Background and Objectives: Previous studies have shown that storage causes RBC membrane damage and subsequent potassium leakage to extracellular environment, with the effects exacerbated by RBC irradiation. While damage to RBC appears to worsen with storage time (ST), ST alone has not been shown to fully account for this phenomenon. It is therefore important to study the extent to which other time-independent factors can affect RBC membrane integrity. RBC mechanical fragility (MF) is evaluated as a surrogate measure of RBC membrane integrity due to its potential to reflect aggregate biochemical and biomechanical changes associated with storage.
Materials and methods: Samples from 45 units non-irradiated and 58 units of irradiated leuko reduced RBC units were subjected to shear stress using a bead mill at different durations at a fixed intensity (50 Hz); induced hemolysis was ascertained via spectral analysis. Profile curves characterized the relationship between stress duration and induced hemolysis, from which specific parameter values were interpolated.
Results: There was high variability among RBC MF parameters. MF profiles were significantly variable among both irradiated and non-irradiated stored RBC units, and in some, within the same units which resulted in distinguishable subpopulations. RBC base-line hemolysis (hemolysis before stress application) MF variation was largely independent of ST. Donor blood type appeared to influence MF parameters and base-line levels.
Conclusion: RBC membrane properties, as defined by MF, vary markedly across RBC units. This variability is largely independent of ST. MF could potentially be used clinically to assess RBC membrane.
Keywords: Red blood cells; Transfusion; Storage lesion; Fragility
Introduction
RBC storage in general results in progressive biochemical and morphological changes collectively known as storage lesion [1,2]. Morphologically, prolonged ex vivo storage results in crenation and spicule formation in RBC, with the consequent development of spheroechinocytes. The spicules are shed as lipid vesicles, leading to spherocytosis and loss of surface-to-volume ratio [3]. Prolonged storage of RBC units also results in progressive depletion of 2,3-diphosphoglycerate (2,3-DPG) levels and subsequent increase in oxygen affinity [4], and depletion of ATP [5] that is linked to energy- dependent cell membrane functions [6,7]. Mechanical properties of the RBC membrane have been shown to be significantly affected in storage lesion, possibly through a loss of endogenous RBC antioxidants that result in oxidative damage to cytoskeletal proteins and membrane phospholipids; this is one manifestation of the plethora of storage- induced changes in RBC membranes which also include membrane phospholipid fasciculation and loss, changes in membrane-bound carbohydrates, and loss of sialic acid related to changes in electro- chemical RBC properties ([8,9] for reviews). These storage-related processes translate into macro effects which – beside changes in cell shape, volume, and density – manifest themselves in increased rigidity of RBC membrane, increased cell agreeability which may contribute to changes in RBC-endothelial interactions, and increased levels of endotoxins and inflammatory cytokines also associated with prolonged blood storage [10,11]. Even though it is well documented in-vitro, the clinical relevance of storage lesion remains controversial. Current debates predominantly focus on whether or not “older” blood may provide lower transfusion efficacy and/or safety [1,12-14]. Similarly, the focus of current clinical research on this topic, as exemplified by th
ABLE [15], RECESS [16] and ARIPI [17,18] studies, remains on the use of storage time (ST) as the sole metric of anticipated RBC performance.
However, focusing on ST as the sole measure of storage lesion ignores the variability in RBC properties that depend on factors other than a blood product’s age. Mean RBC age at donation can span from 38 to 60 days [19] and can influence the nature and magnitude of storage lesion [20,21]. Also notable is that inter-donor variability was shown to be the most significant contributing factor for in-bag (base-line) auto-hemolysis of stored packed RBC [22] and had been identified as one of the major determinants in variability of RBC post-transfusion 51Cr 24 hour in-vivo recovery [23,24]. And variably donor-specific metabolomic changes in pRBC in storage had been highlighted in a recent presentation by Dumont et al. [25]. Hence, the disparity of the results of studies looking to compare “old” versus “new” blood product units may not be so surprising; a true test of storage effects would likely require metrics directly related to physiological properties of RBC. It remains to be shown which if any such in vitro parameter(s) can be reliably correlated with transfusion efficacy and outcomes
RBC mechanical fragility (MF) [27,28] and related flow properties [29] have been suggested as potential candidates to supplement ST as an aggregate metric of RBC storage lesion. In the present study, we investigated the utility of MF as a measure of RBC membrane integrity among RBC units, including irradiated, in Hospital Blood Bank inventories and how this is independent of ST.
*Corresponding author: Abba C. Zubair, Transfusion Medicine and Stem Cell Therapy, Department of Laboratory Medicine and Pathology, Mayo Clinic, FL, USA, Tel: 904-956-3318; Fax: 904-956-3356; E-mail: zubair.abba@mayo.edu
Received April 16, 2013; Accepted May 28, 2013; Published June 05, 2013
Citation: Tarasev M, Alfano K, Chakraborty S, Bertholf M, Zubair A (2013) Mechanical Fragility as a Potential Time-Independent Measure of Membrane Integrity among Stored RBC Units. J Blood Disorders Transf 4:139. doi:10.4172/2155-9864.1000139
Copyright: © 2013 Tarasev M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
J Blood Disorders Transf
ISSN: 2155-9864 JBDT, an open access journal Volume 4 • Issue 2 • 1
These effects may be further exacerbated when RBC are irradiated to prevent the risk of graft versus host disease (GVHD). In the Mayo Clinic (Jacksonville FL) facility, a large number of transfusions are performed to organ transplant and other immune-compromised patients, and as a result all transfused units are irradiated. Longitudinal studies are typically based on a small sample size, and are useful to track changes over time but make it challenging to asses any potential unit-to- unit variability. Because of the above-noted limitations of longitudinal studies, we elected to implement a different approach by assessing multiple diverse units at the point of transfusion. Such an approach makes ST just one potential variable among others. Understanding such cross-inventory variability is particularly important, as this is the variability that will be present at the point of care. It is noteworthy to mention that a recent study reported based on 136 observations an weak (r = -0.18, R2 ~ 0.03) inverse relationship between the age of blood and the increment in hematocrit post transfusion [26]. At the same time presented data show a great variability in the changes in hematocrit even when transfused units were of the same of similar age. With storage time responsible for ~ 3 percent of outcome variability it remains to be determined what the factors responsible for the remaining 97 percent are. It seems that this variability in transfusion outcomes in likely to be better understood through potential variability in stored cells’ physiological properties; with RBC membrane presenting an attractive target for investigation. RBC mechanical fragility (MF) [27,28] and related flow properties [29] have been suggested as potential candidates to supplement ST as an aggregate metric of RBC storage lesion. In the present study, we investigated the utility of MF as a measure of RBC membrane integrity among RBC units, including irradiated, in Hospital Blood Bank inventories and how this is independent of ST.
Materials and Methods
Sample Collection: The RBC test segments used to assess RBC MF were obtained from leukoreduced units released for transfusion at the University of Michigan Hospital Blood Bank of, in case of irradiated units, used for transfusion during liver transplants at Mayo Clinic in Jacksonville, Florida. Units were collected by either whole blood (WB) or apheresis (AP) collection. Both WB and AP non-irradiated units as well as irradiated AP units were stored in AS3, while irradiated WB units were stored in AS5 reflecting the differences in manufacturing practices of different blood supplies. The test segments from the Mayo Clinic were shipped overnight to Dr. Tarasev’s laboratory in Ann Arbor, Michigan. The content of four segments from the same RBC unit was combined for testing to mitigate any potential variability between individual segments. These were diluted to 1.7 g/dl total Hb concentration (as measured by Hemoglobin B system from HemoCue, CA), corresponding to about 4% hematocrit, with AS-3 buffer containing 40 g/L albumin (Sigma). Resultant RBC suspension was gently agitated and a liquotted into 2 ml low-retention centrifuge tubes (Denville, NJ) at 330 μl per tube.
Fragility Test: Mechanical stress was applied to RBC samples with the use of a Tissue Lyser LT (Quiagen, Hilden, Germany) bead mill (at an oscillation frequency of 50 Hz) in the presence of one 7mm diameter stainless steel ball at 22±2 oC. Samples from each RBC unit were subjected to such stress at 10 different durations (ranging from 30 sec to 60 min for non-irradiated and 45 min for irradiated samples) to ensure a wide range of achieved hemolysis levels. Un-lysed cells were precipitated by centrifuging the samples for 5 minutes at 1,300 RPM and aliquots of supernatant were used for spectral analysis
Hemolysis Assessment: Base-line hemolysis (hemolysis prior to the application of mechanical stress) and mechanically induced hemolysis (Hem, with sub-index indicating applied stress duration) were determined based on the difference in absorbance at 576 nm, a wavelength of oxygenated Hb maximum, and a baseline absorbance at 700 nm. It was expressed as a fraction of cell-free hemoglobin (HBF) relative to total hemoglobin concentration (HbT) according to Formula 1 which included the correction for sample hematocrit as detailed by Sowemino-Coker [30]. Total haemoglobin concentration for each diluted RBC sample was determined by subjecting a small (30-40 µL) aliquot to repeated (5x) rapid freeze-thaw using liquid nitrogen. In control experiments, such treatment was shown to fully lyse RBC.
Spectroscopic measurements were performed with NanoDrop N100 spectrophotometer (Thermo Fisher Scientific, MI). Average standard deviation of raw spectrophotometric data points with three independent measurements per each data point, was about six percent
RBC Fragility profiles were described by the hemolysis parameters (Hem-parameters) representing amount of hemolysis achieved as a result of small (0.5-2 min), medium (5-15 min) and large (30-60 min) stress durations at a set stress intensity, corresponding to overall small, medium and large total applied stress magnitude – with each magnitude represented by a corresponding MF metric (i.e., relevant Hem-parameter). Unlike single-point measurements that use single stress duration at a single stress intensity, as implemented e.g. by Raval et al. [28], MF profiles allow to record sample RBC propensity to hemolyse over the range of applied stress magnitudes, from that resulting in minimal to that resulting in nearly total hemolysis of cells in the tested sample, thereby allowing multiple fragility-based indexes to be interpolated for separate analyses [27]. Fragility parameters at particular stress durations were obtained from best fit second- order polynomial regression to the experimental data. For curves exhibiting significant deviations from a simple polynomial, raw data was subdivided into low and high hemolysis sub-sets and the fits were obtained independently for each subset of the data. Statistical Analysis: Data is presented in the form of mean ± standard deviation, or median and range where appropriate. Correlations among parameters were determined using linear regression analysis with ANOVA model (Excel, Microsoft, WA) with significance defined at α=0.05 with Pearson correlation coefficients (r) and significance (p) values reported. Categorical variables were included the models where appropriate. Student t-test with a two-tailed p-value of 0.05 was used to test for statistical significance.
Results
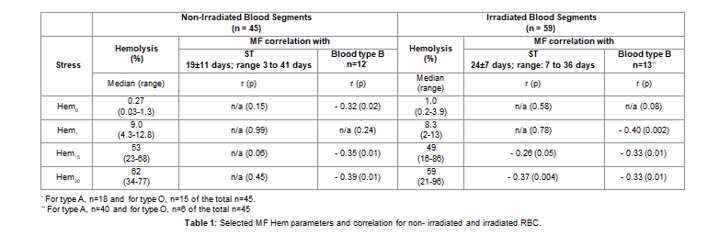
1. RBC units showed significant inter and intra-unit variation of RBC MF profiles and parameters: Both irradiated and non-irradiated Red Blood Cells (RBC) exhibited significant variability in the observed MF profiles, which were grouped into three general categories based on their overall shapes as shown in figure 1. For a small fraction of RBC (profile α) the maximum applied stress was sufficient to achieve close to full hemolysis. For other units (profiles β and δ) 1 hour of applied stress resulted in less than 80% cell being lysed with the RBC of profile β reaching a plateau at about 70-80% of hemolysis (Figure 1). Irradiated and non-irradiated RBC exhibited about equal distribution among these three profile types. A fraction
J Blood Disorders Transf
ISSN: 2155-9864 JBDT, an open access journal Volume 4 • Issue 2 • 1
of the RBC exhibited features which indicated the presence of at least two well-defined “subpopulations” of RBC within the unit – each defined by having its own discernible profile or a potential hemolysis threshold value (profile γ, Figure 1). Furthermore, existence of highly stress-resistant subpopulations should be assumed for all RBCs excluding profile, as significant fraction of cells (20-30 percent in case of profile γ) seem to be able to withstand the maximum applied duration of stress. RBCs showing high resistance to mechanical stress were still able to be lysed via rapid freeze-thaw using liquid nitrogen, or by chemical means as employed by a Hb-meter (HemoCue), which confirms that the observed plateaus were indeed due to unlysed cells. No significant correlation was observed between the ST and the profile categories.
Levels of base-line hemolysis (Hem0) and MF profile- derived induced hemolysis parameters (Hem) also varied significantly among the RBC. Notably the base-line hemolysis values for irradiated RBC were significantly elevated compared to that of non-irradiated (Table 1). However observed differences in MF between irradiated and non-irradiated segments were largely not statistically significant. Overall variability in fragility, as described by Hem parameters, was higher for irradiated RBC: up to 6 fold, compared to 3-4 fold differences for non-irradiated RBC (Table 1).
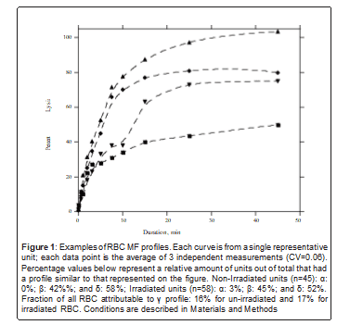
2. Non-irradiated RBC MF parameters were independent of storage time: For both irradiated and non-irradiated RBC storage time ranges from “new” to “old” with age distribution that was close to normal (Table 1). Hem-parameters obtained for non-irradiated RBC exhibited no correlation with the storage time duration (ST) (p>0.05). For irradiated RBC regression analysis indicates a weak negative correlation between ST and Hem-parameters related to larger applied stress, with the maximum dependence observed for H30 at ~10 percent (Table 1). Consistent with the noted poor correlation between RBC MF and ST, units of the same age often responded significantly differently to applied mechanical stress. Conversely, units with very different STs can have substantially similar MF profiles (Figure 2). For irradiated units, storage time after irradiation (STI) can be an additional parameter reflecting quality as previous studies reported faster RBC degradation post-irradiation [31,32]. Surprisingly, we did not observe a statistically significant correlation between STI
and in-bag (base-line) hemolysis. STI in this study was a weak predictor of RBC MF at larger stress magnitudes (increase in Person correlation coefficient for Hem30 from -0.37 to -0.44), but not of induced hemolysis at small or medium stress. It is possible that the correlations would be more pronounced but for a relatively short (9.5 ± 3 days at testing) STI in this study. Multiple factors, beside storage time, can affect the properties of RBC at the time of transfusion. Such factors may potentially include manufacturing methods, storage conditions and donor- specific attributes.
Blood type B was associated with lower propensity to hemolysis: For both irradiated and non-irradiated cells, ABO were found to be correlated to RBC membrane MF with blood type B better able to withstand mechanical stress compared to types A and O blood (Table 1). RBC average STs for the ABO types can differ due to different usage rates. Type B units were on average older for irradiated and younger for non-irradiated units used in this study1. Thus ST and ABO are potentially dependent variables. For non-irradiated RBC, regression analyses on data subsets based on ABO exhibited no correlation between ST and MF parameters. Similar analysis performed on irradiated RBC revealed no correlation in the type B subset, with type A RBC exhibiting the dependence similar to that seen for irradiated samples overall. Regression using ST and type B (as a categorical variable) as independent variables in irradiated RBC show negative correlation to ABO for smaller stress parameters with both ST and ABO being significant independent variables for larger MF parameters (r ~ -0.45, p<0.05). These observations confirm that ABO, specifically type B 1ST for blood type B vs. blood types A and O for non- irradiated: 14 ± 6 vs. 22 ± 12 and 20 ± 13 days; irradiated: 27 ± 9 vs. 17± 5 and 18 ± 6 days compared to types A and O is indeed a storage time-independent predictor of RBC MF. This conclusion does not preclude ABO being an extraneous variable correlated to some unaccounted for parameter(s)
Discussion
It is known that RBC units from different donors differ in their biochemical and biomechanical properties, in part due to in vivo age upon collection which can affect cell’s membrane properties [21], with differences ranging from cell size to membrane composition. It is also known that red cell senescence brings about a decrease in plasticity of the cell membrane [33,34], which may likewise be a donor-
J Blood Disorders Transf
ISSN: 2155-9864 JBDT, an open access journal Volume 4 • Issue 2 • 1
specific phenomenon [35]. However, it has not yet been shown how extensive such differences can be, nor that such intrinsic variability in cell properties could be detectable not only across different units but also within a single donated unit. In particular, these issues are especially noteworthy with regard to irradiated units due to their higher propensity for damage; hence, they are compared here at their respective points of transfusion.
Although sub-populations of RBC had been reported and investigated before, most commonly they are discussed in terms of RBC younger/older fractions (representing cells of different metabolic age). While such fractions may indeed differ in cell’s size, volume, Hb concentration and notably ability to deform [36], a smooth MF distribution would be expected in healthy donors. Appearance of RBC sub- populations which differ in membrane mechanical properties can be expected and indeed had been reported for subjects with pathological conditions like sickle cell anemia, elliptocytosis or sepsis although Dobbe et.al. [37] noted an existence of fractions, variable from zero to 20 percent, of both hypo- and hyper-deformable cells even in normal donors. The existence of RBC subpopulations with significantly different abilities to withstand applied mechanical stress is clearly demonstrated by the profile curve representing category γ in figure 2. To a lesser extent, it is also exhibited by profile δ, figure 2. Additionally, as noted above, the plateau of profile β suggests that in this category there exists a small (approx. 20%) but well-defined fraction of cells with a much lower propensity for lysis than the rest. Moreover, it is possible that at least all units may also have RBC subpopulations with varying stress resistances, which are not well resolved with the employed granularity of this study.
MF of RBC membranes is commonly described by a Mechanical Fragility Index (MFI) which represents the amount of hemolysis resulting from a predetermined stress [28,39]. Those would be analogous to one of the multiple HEM parameters used in the present study. Data presented here indicate that, by themselves, such single-point metrics may not fully characterize RBC ability to withstand mechanical stress. Wide differences in profile slope and shape, including the existenc
of distinct sub-populations within particular units, demonstrates the value in evaluating RBC properties over a range of applied stresses. Further study is necessary to determine which single or combination of stress magnitudes correlate with clinical performance. In this study, regression analysis indicate potentially different impact of ABO groups on RBC storability with type B blood on average being less fragile, than that of types A and O. Further studies, specifically designed to confirm this observation would be necessary; however as the mechanisms for such correlation, if indeed present, are not immediately apparent, it is possible that ABO itself is in part a proxy for as-yet undetermined donor or manufacturing-specific factors. It is well-documented that irradiation results in increased base-line hemolysis of RBC [40, 41]. Base-line hemolysis was also shown to gradually increase with ST while also being significantly dependent on the type of storage solution used and method of leukoreduction, while being poorly correlated with unit hematocrit [22,42]. The values for base-line hemolysis observed in the present study were on average comparable with those reported previously however, especially in case of irradiated samples, there were outliers which exhibited exceptionally high hemolysis levels. Similar to low-stress Hem- parameters, base-line hemolysis was found to be independent of storage time (Table 1). It could thus be that a correlation between base-line hemolysis and longer STs was not detected due to the background of high unit-to-unit variability.
The main limitation of the current study was a logistically- necessitated reliance upon test segments. That leaves open the question of equivalency between cells’ properties in a unit versus in its test segments even though segments in this study were equivalent to their units in storage solution composition. Potential differences in physiological properties and storability of RBC between units and segments had not been adequately addressed in peer-reviewed literature to date and are limited to analysis of auto-hemolysis. Published data, based on very limited sample sizes, indicate that segments may potentially differ from the units and from each other in auto-hemolysis levels [43]. Present study alleviated any potential segment-to-segment variability by using a mixture of four segments for the testing. It should also be noted that it remains to be shown whether base-line hemolysis, especially at low levels, is significantly indicative of the physiological state of RBC. While it remains to be understood how RBC physiological properties in units may differ from that in the unit segments, we believe that segments ca
J Blood Disorders Transf
ISSN: 2155-9864 JBDT, an open access journal Volume 4 • Issue 2 • 1
still provide an initial “snapshot” of the variability in RBC properties of different units. We submit that the uniformity of processing allows for a meaningful comparison among units and allows drawing some preliminary conclusions on unit-to-unit variability.
It was previously proposed [27,28] that MF can potentially provide a standard for quantifying RBC membrane integrity during storage. Rapid and inexpensive tests for MF parameters, presently under development, could become a useful tool in clinical practice. For example, individualized unit data regarding levels and rates of RBC lesion could enable a quality-based approach to inventory management (similar to that used for perishable foods [44]) instead of relying upon ST alone as exemplified by the use of First-In-First-Out method. For example, a unit whose prospective efficacy is declining more rapidly than others may get used sooner, to minimize any quality loss. In summary, we describe and compare here various MF-based parameters for characterizing stored irradiated RBC units. Our main focus was on MF profiles constructed by varying one stress parameter and tracking the resultant effect upon percentage hemolysis, and select MF parameters derived from these profiles. This study demonstrated the existence of distinct cell subpopulations in blood from healthy donors, which is characterized by distinguishable MF profiles within individual RBC units. It also presents evidence for substantial independence of stored RBC unit-to-unit MF variability from ST. Subsequent work will further investigate factors potentially affecting MF and its variability in blood bank inventories, as well as correlations of in vitro MF parameters to in vivo cell performance and clinical outcomes
References
- van de Watering L (2011) Red cell storage and prognosis. Vox Sang 100: 36- 45.
- Hess JR (2010) Red cell changes during storage. Transfus Apher Sci 43: 51-59.
- Card RT (1988) Red cell membrane changes during storage. Transfus Med Rev 2: 40-47.
- Högman CF, Knutson F, Lööf H, Payrat JM (2002) Improved maintenance of 2,3 DPG and ATP in RBCs stored in a modified additive solution. Transfusion 42: 824-829.
- Högman CF, Knutson F, Lööf H, Payrat JM (2002) Improved maintenance of 2,3 DPG and ATP in RBCs stored in a modified additive solution. Transfusion 42: 824-829.
- Haradin AR, Weed RI, Reed CF (1969) Changes in physical properties of stored erythrocytes relationship to survival in vivo. Transfusion 9: 229-237.
- Bartlett GR: Red cell metabolism: review highlighting changes during storage; in Greenwalt TJ, Jamieson GA, (eds) The Human Red Cell In Vitro. New York, Grune & Stratton, Inc., 1973: p. 5-28.
- Almac E, Ince C (2007) The impact of storage on red cell function in blood transfusion. Best Pract Res Clin Anaesthesiol 21: 195-208. 9
- Ho J, Sibbald WJ, Chin-Yee IH (2003) Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med 31: S687-697.
- Holme S (2005) Current issues related to the quality of stored RBCs. Transfus Apher Sci 33: 55-61.
- Spinella PC, Sparrow RL, Hess JR, Norris PJ (2011) Properties of stored red blood cells: understanding immune and vascular reactivity. Transfusion 51: 894-900.
- Zubair AC (2010) Clinical impact of blood storage lesions. Am J Hematol 85: 117-122.
- Triulzi DJ, Yazer MH (2010) Clinical studies of the effect of blood storage on patient outcomes. Transfus Apher Sci 43: 95-106.
- Spinella PC, Doctor A, Blumberg N, Holcomb JB (2011) Does the storage duration of blood products affect outcomes in critically ill patients? Transfusion 51: 1644-1650.
- Lacroix J, Hébert P, Fergusson D, Tinmouth A, Blajchman MA, et al. (2011) The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev 25: 197-205.
- Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, et al. (2010) Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7). Transfus Apher Sci 43: 107-116.
- Fergusson D, Hutton B, Hogan DL, LeBel L, Blajchman MA, et al. (2009) The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfus Med Rev 23: 55-61.
- Fergusson DA, Hébert P, Hogan DL, LeBel L, Rouvinez-Bouali N, et al. (2012) Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA 308: 1443- 1451.
- Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, et al. (2008) Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 112: 4284-4291.
- Sparrow RL, Healey G, Patton KA, Veale MF (2006) Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus Apher Sci 34: 15-23.
- Sparrow RL, Veale MF, Healey G, Payne KA (2007) Red blood cell (RBC) age at collection and storage influences RBC membrane-associated carbohydrates and lectin binding. Transfusion 47: 966-968.
- Hess JR, Sparrow RL, van der Meer PF, Acker JP, Cardigan RA, et al. (2009) Red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion 49: 2599- 2603.
- Dern RJ, Gwinn RP, Wiorkowski JJ (1966) Studies on the preservation of human blood. I. Variability in erythrocyte storage characteristics among healthy donors. J Lab Clin Med 67: 955-965.
- Dumont LJ, AuBuchon JP (2008) Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 48: 1053-1060.
- Dumont LJ, Zimring JC, Roback JD: Correlation of RBC Metabolomic Changes During Storage with RBC Survival After Transfusion in Human Autologous Donor/Recipients, AABB Conference 2012. Boston, MA,. Transfusion. 2012;52 12A.
- Pieracci FM, Moore EE, Chin T, Townsend N, Gonzalez E, et al. (2012) The age of transfused blood predicts hematocrit response among critically ill surgical patients. Am J Surg 204: 269-273.
- . Alfano K, Tarasev M: Investigating Direct Non-Age Metrics of Stored Blood Quality Loss. The Internet Journal of Medical Technology. 2011;5.
- Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, et al. (2010) The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang 99: 325-331.
- Barshtein G, Manny N, Yedgar S (2011) Circulatory risk in the transfusion of red blood cells with impaired flow properties induced by storage. Transfus Med Rev 25: 24-35.
- Sowemimo-Coker SO (2002) Red blood cell hemolysis during processing. Transfus Med Rev 16: 46-60.
- Moroff G, Holme S, AuBuchon JP, Heaton WA, Sweeney JD, et al. (1999) Viability and in vitro properties of AS-1 red cells after gamma irradiation. Transfusion 39: 128-134.
- Koziczak R, Gonciarz M, Krokosz A, Szweda-Lewandowska Z (2003) The influence of split doses of gamma-radiation on human erythrocytes. J Radiat Res 44: 217-222.
- Farges E, Grebe R, Baumann M (2002) Viscoelastic and biochemical properties of erythrocytes during storage with SAG-M at +4 degrees C. Clin Hemorheol Microcirc 27: 1-11.
- Li-de X, Li G, Zong-Yi Y, Wei-Juan Y, Da-Gong S, et al. (2003) The microrheological changes in the course of erythrocyte senescence after phenylhydrazine injection. Clin Hemorheol Microcirc 28: 5-11.
J Blood Disorders Transf
ISSN: 2155-9864 JBDT, an open access journal Volume 4 • Issue 2 • 1
still provide an initial “snapshot” of the variability in RBC properties of different units. We submit that the uniformity of processing allows for a meaningful comparison among units and allows drawing some preliminary conclusions on unit-to-unit variability.
It was previously proposed [27,28] that MF can potentially provide a standard for quantifying RBC membrane integrity during storage. Rapid and inexpensive tests for MF parameters, presently under development, could become a useful tool in clinical practice. For example, individualized unit data regarding levels and rates of RBC lesion could enable a quality-based approach to inventory management (similar to that used for perishable foods [44]) instead of relying upon ST alone as exemplified by the use of First-In-First-Out method. For example, a unit whose prospective efficacy is declining more rapidly than others may get used sooner, to minimize any quality loss. In summary, we describe and compare here various MF-based parameters for characterizing stored irradiated RBC units. Our main focus was on MF profiles constructed by varying one stress parameter and tracking the resultant effect upon percentage hemolysis, and select MF parameters derived from these profiles. This study demonstrated the existence of distinct cell subpopulations in blood from healthy donors, which is characterized by distinguishable MF profiles within individual RBC units. It also presents evidence for substantial independence of stored RBC unit-to-unit MF variability from ST. Subsequent work will further investigate factors potentially affecting MF and its variability in blood bank inventories, as well as correlations of in vitro MF parameters to in vivo cell performance and clinical outcomes
References
- van de Watering L (2011) Red cell storage and prognosis. Vox Sang 100: 36- 45.
- Hess JR (2010) Red cell changes during storage. Transfus Apher Sci 43: 51-59.
- Card RT (1988) Red cell membrane changes during storage. Transfus Med Rev 2: 40-47.
- Högman CF, Knutson F, Lööf H, Payrat JM (2002) Improved maintenance of 2,3 DPG and ATP in RBCs stored in a modified additive solution. Transfusion 42: 824-829.
- Högman CF, Knutson F, Lööf H, Payrat JM (2002) Improved maintenance of 2,3 DPG and ATP in RBCs stored in a modified additive solution. Transfusion 42: 824-829.
- Haradin AR, Weed RI, Reed CF (1969) Changes in physical properties of stored erythrocytes relationship to survival in vivo. Transfusion 9: 229-237.
- Bartlett GR: Red cell metabolism: review highlighting changes during storage; in Greenwalt TJ, Jamieson GA, (eds) The Human Red Cell In Vitro. New York, Grune & Stratton, Inc., 1973: p. 5-28.
- Almac E, Ince C (2007) The impact of storage on red cell function in blood transfusion. Best Pract Res Clin Anaesthesiol 21: 195-208. 9
- Ho J, Sibbald WJ, Chin-Yee IH (2003) Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med 31: S687-697.
- Holme S (2005) Current issues related to the quality of stored RBCs. Transfus Apher Sci 33: 55-61.
- Spinella PC, Sparrow RL, Hess JR, Norris PJ (2011) Properties of stored red blood cells: understanding immune and vascular reactivity. Transfusion 51: 894-900.
- Zubair AC (2010) Clinical impact of blood storage lesions. Am J Hematol 85: 117-122.
- Triulzi DJ, Yazer MH (2010) Clinical studies of the effect of blood storage on patient outcomes. Transfus Apher Sci 43: 95-106.
- Spinella PC, Doctor A, Blumberg N, Holcomb JB (2011) Does the storage duration of blood products affect outcomes in critically ill patients? Transfusion 51: 1644-1650.
- Lacroix J, Hébert P, Fergusson D, Tinmouth A, Blajchman MA, et al. (2011) The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev 25: 197-205.
- Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, et al. (2010) Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7). Transfus Apher Sci 43: 107-116.
- Fergusson D, Hutton B, Hogan DL, LeBel L, Blajchman MA, et al. (2009) The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfus Med Rev 23: 55-61.
- Fergusson DA, Hébert P, Hogan DL, LeBel L, Rouvinez-Bouali N, et al. (2012) Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA 308: 1443- 1451.
- Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, et al. (2008) Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 112: 4284-4291.
- Sparrow RL, Healey G, Patton KA, Veale MF (2006) Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus Apher Sci 34: 15-23.
- Sparrow RL, Veale MF, Healey G, Payne KA (2007) Red blood cell (RBC) age at collection and storage influences RBC membrane-associated carbohydrates and lectin binding. Transfusion 47: 966-968.
- Hess JR, Sparrow RL, van der Meer PF, Acker JP, Cardigan RA, et al. (2009) Red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion 49: 2599- 2603.
- Dern RJ, Gwinn RP, Wiorkowski JJ (1966) Studies on the preservation of human blood. I. Variability in erythrocyte storage characteristics among healthy donors. J Lab Clin Med 67: 955-965.
- Dumont LJ, AuBuchon JP (2008) Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 48: 1053-1060.
- Dumont LJ, Zimring JC, Roback JD: Correlation of RBC Metabolomic Changes During Storage with RBC Survival After Transfusion in Human Autologous Donor/Recipients, AABB Conference 2012. Boston, MA,. Transfusion. 2012;52 12A.
- Pieracci FM, Moore EE, Chin T, Townsend N, Gonzalez E, et al. (2012) The age of transfused blood predicts hematocrit response among critically ill surgical patients. Am J Surg 204: 269-273.
- . Alfano K, Tarasev M: Investigating Direct Non-Age Metrics of Stored Blood Quality Loss. The Internet Journal of Medical Technology. 2011;5.
- Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, et al. (2010) The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang 99: 325-331.
- Barshtein G, Manny N, Yedgar S (2011) Circulatory risk in the transfusion of red blood cells with impaired flow properties induced by storage. Transfus Med Rev 25: 24-35.
- Sowemimo-Coker SO (2002) Red blood cell hemolysis during processing. Transfus Med Rev 16: 46-60.
- Moroff G, Holme S, AuBuchon JP, Heaton WA, Sweeney JD, et al. (1999) Viability and in vitro properties of AS-1 red cells after gamma irradiation. Transfusion 39: 128-134.
- Koziczak R, Gonciarz M, Krokosz A, Szweda-Lewandowska Z (2003) The influence of split doses of gamma-radiation on human erythrocytes. J Radiat Res 44: 217-222.
- Farges E, Grebe R, Baumann M (2002) Viscoelastic and biochemical properties of erythrocytes during storage with SAG-M at +4 degrees C. Clin Hemorheol Microcirc 27: 1-11.
- Li-de X, Li G, Zong-Yi Y, Wei-Juan Y, Da-Gong S, et al. (2003) The microrheological changes in the course of erythrocyte senescence after phenylhydrazine injection. Clin Hemorheol Microcirc 28: 5-11.
J Blood Disorders Transf
ISSN: 2155-9864 JBDT, an open access journal Volume 4 • Issue 2 • 1
- Landaw SA: Factors that accelerate or retard red blood cell senescence. Blood Cells. 1988;14: 47-67.
- Gifford SC, Derganc J, Shevkoplyas SS, Yoshida T, Bitensky MW (2006) A detailed study of time-dependent changes in human red blood cells: from reticulocyte maturation to erythrocyte senescence. Br J Haematol 135: 395- 404.
- Dobbe JG, Hardeman MR, Streekstra GJ, Strackee J, Ince C, et al. (2002) Analyzing red blood cell-deformability distributions. Blood Cells Mol Dis 28: 373-384.
- Condon MR, Kim JE, Deitch EA, Machiedo GW, Spolarics Z (2003) Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am J Physiol Heart Circ Physiol 284: H2177-2184.
- Kameneva MV, Antaki JF: Mechanical Trauma to Blood; in Baskurt OK, (ed) Handbook of Hemorheology and Hemodynamics. Amsterdam, IOS Press, Netherlands, 2007: p. 206-227. Hauck B, Oremek D, Zimmermann R, Ruppel R, Troester B, et al. (2011) Influence of irradiation on in vitro red-blood-cell (RBC) storage variables of leucoreduced RBCs in additive solution PAGGS-M. Vox Sang 101: 21-27.
- Zimmermann R, Schoetz AM, Frisch A, Hauck B, Weiss D, et al. (2011) Influence of late irradiation on the in vitro RBC storage variables of leucoreduced RBCs in SAGM additive solution. Vox Sang 100: 279-284.
- Gammon RR, Strayer SA, Avery NL, Mintz PD (2000) Hemolysis during leukocyte-reduction filtration of stored red blood cells. Ann Clin Lab Sci 30: 195-199.
- Janatpour KA, Paglieroni TG, Crocker VL, DuBois DJ, Holland PV (2004) Visual assessment of hemolysis in red blood cell units and segments can be deceptive. Transfusion 44: 984-989.
- i>Wells JH, Singh RP: A quality-based inventory issue policy for perishable foods.Journal of Food Processing and Preservation. 1989;12: 271-292.
J Blood Disorders Transf
ISSN: 2155-9864 JBDT, an open access journal Volume 4 • Issue 2 • 1


